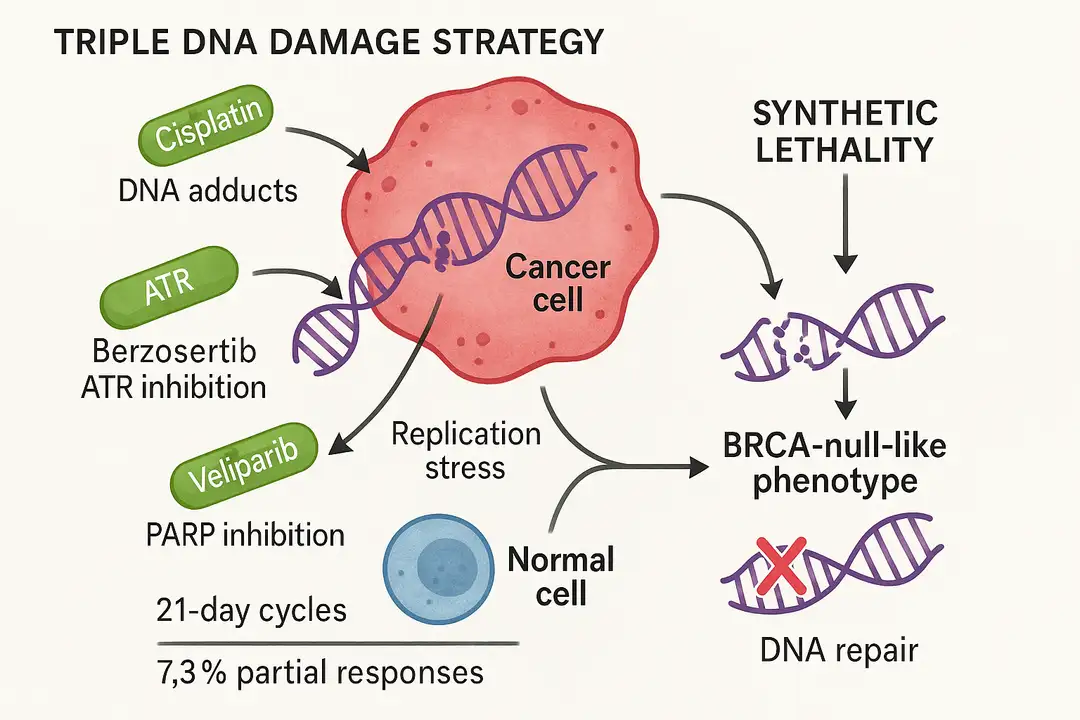
The Strategy
This phase I trial tested a novel approach to create a "BRCA null-like" phenotype by combining three DNA-targeting agents: cisplatin (DNA-damaging), berzosertib (ATR inhibitor), and veliparib (PARP inhibitor). The goal was to overwhelm cancer cells' DNA repair capacity.
Study Design
Design: Open-label, 3+3 dose escalation trial Population: 53 patients with refractory solid tumors Regimen: 21-day cycles with:
- Cisplatin IV on days 1 and 8
- Berzosertib IV on days 2 and 9
- Veliparib oral twice daily on days 1-3 and 8-10
Previous therapy: Platinum and PARP inhibitor pre-treatment was permitted
Maximum Tolerated Dose
Recommended Phase II Dose:
- Cisplatin: 40 mg/m² on days 1/8
- Berzosertib: 210 mg/m² on days 2/9
- Veliparib: 200 mg twice daily on days 1-3 and 8-10
Efficacy Results
Response Rate: 3 confirmed partial responses (7.3% of 41 evaluable patients) Additional responses: 2 unconfirmed partial responses Treatment duration: Median 4 cycles (range: 1-25) Dose modifications: 66% of patients required at least one dose reduction
Safety Profile
Most common grade 3/4 toxicities (myelosuppressive):
- Anemia: 37.7%
- Thrombocytopenia: 32.1%
- Leukopenia: 24.5%
- Neutropenia: 22.6%
- Lymphopenia: 20.8%
Safety assessment: No new safety signals beyond expected myelosuppression
Mechanistic Insights
Pharmacodynamic findings: Adding berzosertib to veliparib/cisplatin increased RAD51-positive tumor cells in BRCA-wildtype specimens, indicating enhanced DNA damage response and increased replication stress.
Clinical Significance
This triple combination demonstrates:
- Antitumor activity in heavily pretreated patients
- Activity in both homologous recombination-deficient and proficient tumors
- Responses in platinum-pretreated patients
- Proof-of-concept for synthetic lethality approaches
Future Directions
The established MTD and evidence of enhanced DNA damage response support phase II development. The combination may be particularly valuable for tumors with intact DNA repair mechanisms that resist single-agent approaches.
Innovation Impact
This study validates the concept of creating synthetic BRCA deficiency through combination DNA damage response inhibition, potentially expanding treatment options for patients with refractory solid tumors lacking inherent DNA repair defects.
Editorial note: This content was developed with the support of artificial intelligence technologies to optimize the writing and structuring of the information. All material was carefully reviewed, validated, and supplemented by human experts prior to publication, ensuring scientific accuracy and adherence to good editorial practices.
#DNARepair #SyntheticLethality #Berzosertib #PARPInhibitors #RefractoryCancers
Sources
- O'Sullivan Coyne, G., et al. (2025). Safety and Tolerability of Berzosertib, an Ataxia-Telangiectasia Related Inhibitor, and Veliparib, an Oral Poly (ADP-ribose) Polymerase Inhibitor, in Combination With Cisplatin in Patients With Refractory Solid Tumors. JCO Precision Oncology, 9, e2500055. https://doi.org/10.1200/PO-25-00055.
Highlights
TribeMD
ASCO GU® 2026

Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care

TribeMD
ASCO GU® 2026

Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care


