Arrhythmias represent one of the most devastating complications following myocardial infarction, significantly increasing patient mortality through mechanisms that have remained poorly understood. While much attention has focused on the ischemic damage to working myocardium, the fate of the cardiac conduction system—particularly the specialized His-Purkinje network responsible for coordinating ventricular contractions—has been largely overlooked. This groundbreaking study published in Nature Cardiovascular Research reveals how the regenerative capacity of neonatal hearts extends to the electrical conduction system, offering unprecedented insights into why some hearts develop fatal arrhythmias while others maintain normal rhythm after injury.
The cardiac conduction system represents one of the most sophisticated electrical networks in biology, orchestrating approximately three billion synchronized heartbeats throughout an average human lifetime. The electrical impulse originates in the sinoatrial node and propagates sequentially through the atria, atrioventricular node, bundle branches, and finally through the His-Purkinje network to coordinate ventricular contraction. When myocardial infarction disrupts this intricate system, the consequences extend far beyond simple muscle damage, creating the substrate for life-threatening arrhythmias including heart block and ventricular dyssynchrony.
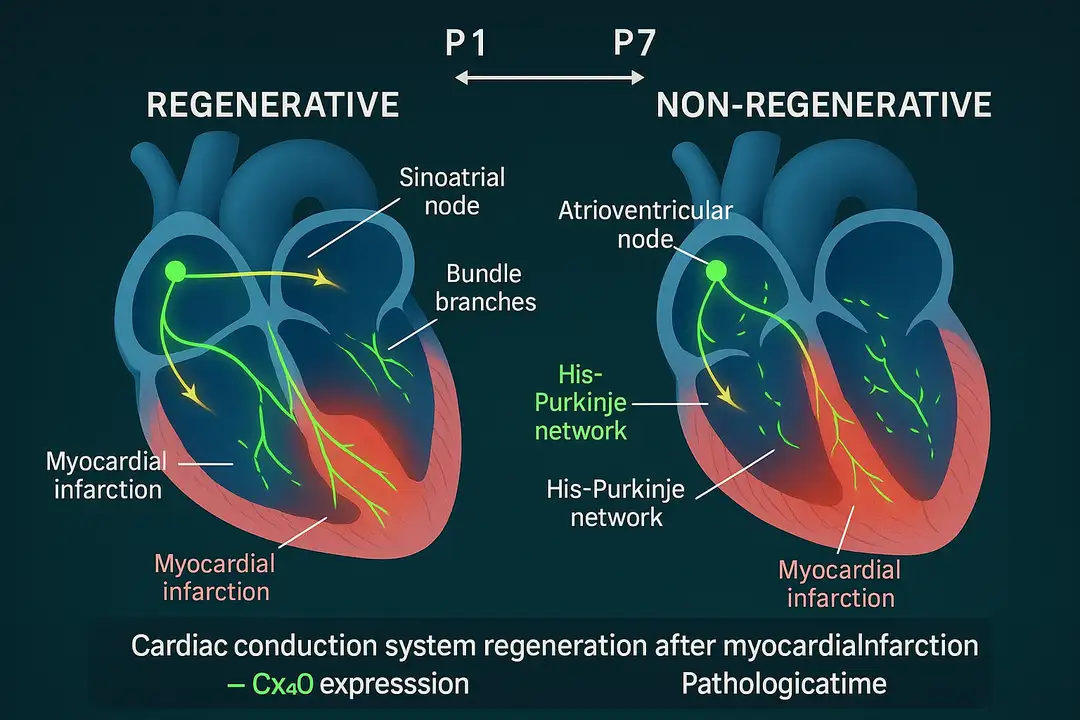
Using advanced tissue-clearing techniques and whole-organ imaging, researchers demonstrated that neonatal mouse hearts possess a remarkable ability to regenerate their conduction systems following injury, while adult hearts undergo pathological remodeling that predisposes to arrhythmias. The study utilized Cx40-eGFP reporter mice to visualize the ventricular conduction system with unprecedented clarity, revealing that the His-Purkinje network undergoes substantial growth and maturation during the first postnatal week. This developmental window coincides precisely with the known regenerative capacity of neonatal mammalian hearts, suggesting that conduction system repair represents an integral component of cardiac regeneration.
The researchers induced myocardial infarction through left anterior descending coronary artery ligation in both regenerative postnatal day 1 (P1) hearts and non-regenerative postnatal day 7 (P7) hearts. Three-dimensional reconstruction revealed that both age groups initially suffered similar disruption to their His-Purkinje networks, with discontinuous fibers and loss of connexin 40 expression proximal to the injury site. However, the subsequent repair responses diverged dramatically between regenerative and non-regenerative hearts, establishing fundamentally different electrical substrates.
Results
Single-cell RNA sequencing of fluorescence-sorted conduction system cells revealed distinct molecular signatures underlying regenerative versus pathological repair. In regenerative P1 hearts, the His-Purkinje network demonstrated robust upregulation of connexin 40 expression in regions distal to the injury, accompanied by enhanced fiber bundling and restoration of normal network architecture. The molecular analysis identified specific transcriptional programs driving conduction system regeneration, including upregulation of developmental transcription factors and ion channel genes essential for fast electrical conduction.
In contrast, P7 non-regenerative hearts exhibited persistent downregulation of connexin 40, disrupted fiber bundling, and disorganized network architecture. The transcriptional signature revealed activation of fibrotic pathways and inflammatory responses that impaired proper conduction system repair. Electrophysiological mapping demonstrated that these molecular changes translated into functional consequences, with regenerative hearts maintaining normal electrical conduction while non-regenerative hearts developed significant conduction delays and abnormal activation patterns.
The study's most striking finding emerged from longitudinal electrophysiological analysis across the regenerative window. Hearts injured at P1 initially showed conduction abnormalities similar to P7 hearts but progressively restored normal electrical function over the subsequent weeks. By contrast, P7 hearts that initially appeared to recover electrically later developed progressive conduction delays and arrhythmogenic substrates. This temporal pattern suggests that the regenerative window for conduction system repair extends beyond the acute injury phase, with ongoing remodeling determining long-term electrical outcomes.
Computational modeling using human cardiac electrophysiology data provided crucial translational insights. When the researchers incorporated the observed conduction system defects from non-regenerative mouse hearts into models of human ventricular activation, they reproduced the characteristic patterns of heart block and bundle branch block commonly observed in post-infarction patients. This modeling approach validated the clinical relevance of the mouse findings and suggested that conduction system damage represents a primary mechanism underlying post-infarction arrhythmias in humans.
Clinical Implications
These findings fundamentally reshape our understanding of post-infarction arrhythmogenesis and point toward novel therapeutic strategies. The demonstration that conduction system regeneration prevents arrhythmias suggests that therapies aimed at enhancing this repair process could significantly improve outcomes for myocardial infarction patients. Current clinical approaches focus primarily on revascularization and working myocardium protection, with limited attention to conduction system preservation or repair.
The identification of specific molecular pathways driving conduction system regeneration opens new avenues for therapeutic intervention. The upregulation of connexin 40 and restoration of proper fiber bundling in regenerative hearts suggest that pharmacological approaches targeting these pathways could enhance conduction system repair in adult hearts. Similarly, the transcriptional programs identified through single-cell sequencing provide potential targets for regenerative therapies.
The temporal dynamics of conduction system repair have important implications for clinical monitoring and intervention timing. The finding that electrical abnormalities can develop progressively after the acute injury phase suggests that long-term electrophysiological monitoring may be necessary to identify patients at risk for late arrhythmic complications. This could inform decisions about prophylactic device implantation or targeted anti-arrhythmic therapies.
The computational modeling results provide a mechanistic framework for understanding why certain post-infarction patients develop heart block or bundle branch block while others maintain normal conduction. This insight could lead to improved risk stratification algorithms that incorporate conduction system assessment alongside traditional measures of left ventricular function and scar burden.
Conclusion
This study establishes conduction system regeneration as a critical determinant of post-infarction electrical stability, revealing why neonatal hearts maintain normal rhythm while adult hearts develop arrhythmogenic substrates after injury. The demonstration that His-Purkinje network repair represents an active biological process, rather than passive healing, fundamentally changes our conceptual framework for understanding post-infarction arrhythmias. The identification of specific molecular mechanisms driving conduction system regeneration provides a roadmap for developing therapies that could restore the heart's natural electrical coordination after injury.
The translational potential of these findings extends beyond basic scientific understanding to practical clinical applications. By elucidating the cellular and molecular basis of conduction system repair, this research opens new therapeutic avenues that could prevent the arrhythmic complications that claim thousands of lives annually. The integration of advanced imaging, single-cell genomics, and computational modeling demonstrates the power of multidisciplinary approaches to solve complex cardiovascular problems.
Perhaps most importantly, this work reveals that the heart's electrical system possesses an intrinsic capacity for regeneration that is lost during postnatal development. Understanding how to reactivate these regenerative programs in adult hearts could transform the treatment of post-infarction patients, shifting the paradigm from managing arrhythmic complications to preventing them through conduction system restoration. As we continue to unravel the mechanisms of cardiac regeneration, the prospect of truly regenerative therapies for heart disease moves closer to clinical reality.
Editorial note: This content was developed with the support of artificial intelligence technologies to optimize the writing and structuring of the information. All material was carefully reviewed, validated, and supplemented by human experts prior to publication, ensuring scientific accuracy and adherence to good editorial practices.
#PostMIManagement #HeartBlock #VentricularDyssynchrony #ArrhythmiaPrevention
#HisPurkinjeNetwork #CardiacRegeneration
Sources
- Sayers, J.R., et al. (2025). Cardiac conduction system regeneration prevents arrhythmias after myocardial infarction. Nature Cardiovascular Research, 4, 163-179.
Highlights
Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care

Medical Affairs
META-AF: Metformin as an Adjunctive Therapy to Catheter Ablation of Atrial Fibrillation

Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care

Medical Affairs
META-AF: Metformin as an Adjunctive Therapy to Catheter Ablation of Atrial Fibrillation


