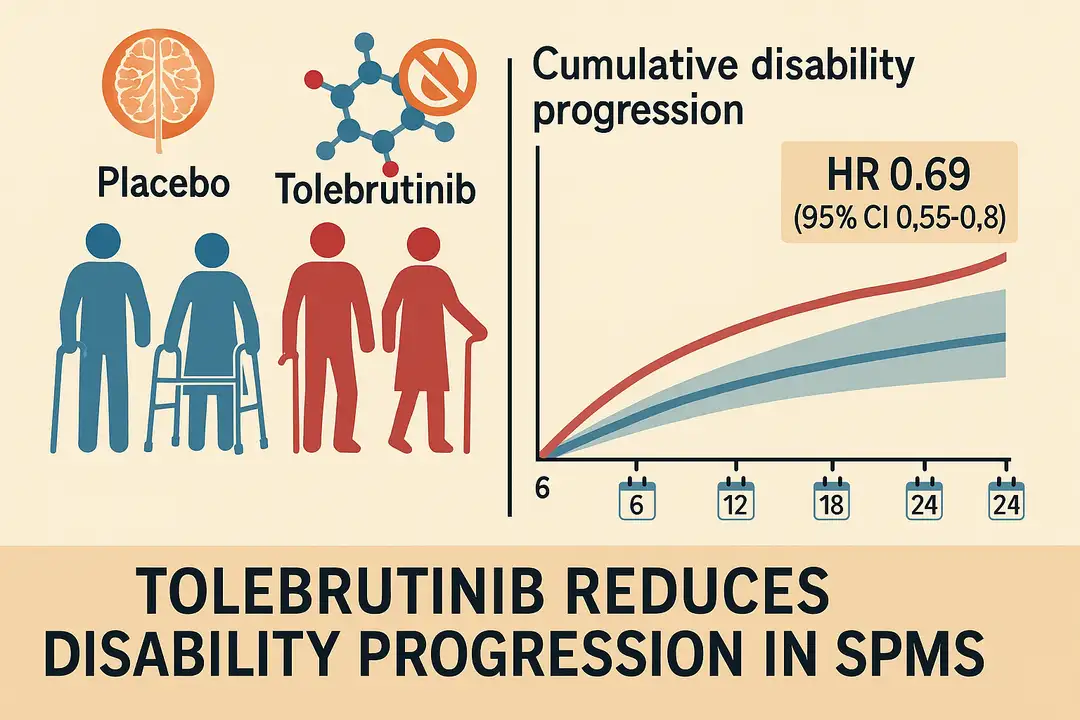
In the phase 3 HERCULES trial, published in The New England Journal of Medicine (2025), tolebrutinib, an oral, brain-penetrant Bruton’s tyrosine kinase (BTK) inhibitor, demonstrated superior efficacy over placebo in reducing disability progression in patients with nonrelapsing secondary progressive multiple sclerosis (SPMS)—a population for whom no approved treatments currently exist.
The trial randomized 1,131 participants in a 2:1 ratio to receive tolebrutinib (60 mg daily) or placebo. Over a median follow-up of 133 weeks, the incidence of 6-month confirmed disability progression was 22.6% with tolebrutinib versus 30.7% with placebo (hazard ratio, 0.69; 95% CI, 0.55–0.88; p=0.003). Notably, all disability events were confirmed as independent of relapse activity.
Key secondary endpoints also favored tolebrutinib, including 3-month confirmed disability progression (HR 0.76), reduction in new/enlarging T2 lesions (relative rate 0.62), and increased likelihood of sustained disability improvement (HR 1.88). Safety data showed a higher rate of adverse events in the tolebrutinib group (81.5% vs. 78.1%), including liver enzyme elevations >3x ULN in 4.0% of patients, leading to one case of liver failure. Following implementation of weekly liver monitoring during the first 12 weeks, no additional severe hepatotoxic events were reported.
These findings support the use of tolebrutinib as a novel disease-modifying therapy capable of slowing disability progression in nonrelapsing SPMS, addressing the unmet need for effective intervention in a historically underserved population.
Editorial note: This content was developed with the support of artificial intelligence technologies to optimize the writing and structuring of the information. All material was carefully reviewed, validated, and supplemented by human experts prior to publication, ensuring scientific accuracy and adherence to good editorial practices.
#SPMS #Tolebrutinib #MultipleSclerosis #BTKinhibitor #NEJM
Sources
- Fox RJ, Bar-Or A, Traboulsee A, et al. Tolebrutinib in nonrelapsing secondary progressive multiple sclerosis. N Engl J Med. 2025;392(19):1883–92. doi:10.1056/NEJMoa2415988
Highlights
TribeMD
ASCO GU® 2026

Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care
TribeMD
ASCO GU® 2026

Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care


