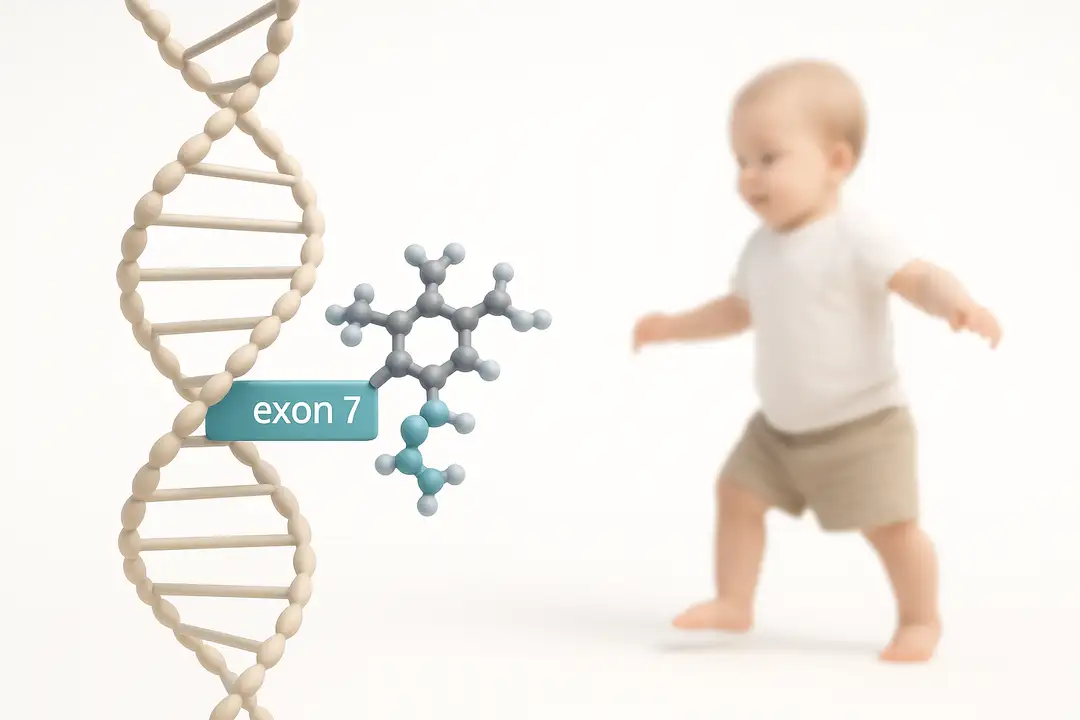
In an editorial accompanying the phase 2 trial by Finkel et al., Sumner reviews presymptomatic use of risdiplam—an oral SMN2 pre‑mRNA splicing modifier—for spinal muscular atrophy (SMA). Risdiplam binds SMN2 transcripts at intron 7 and exon 7, stabilizing exon 7 inclusion and increasing production of full‑length SMN protein, with broad tissue biodistribution and blood–brain barrier penetration.
The study enrolled 26 infants identified largely by newborn screening (8 with two SMN2 copies; 18 with ≥3 copies). At 12 months, 96% could sit unsupported for 5 seconds and 81% for 30 seconds. At 24 months, among 23 children still in follow‑up, all were alive and 81% were walking independently. Six of 26 manifested clinical SMA during the study; all six had two SMN2 copies, consistent with a more severe phenotype.
Timing appears critical. Across approved SMN‑inducing therapies (risdiplam, nusinersen, onasemnogene abeparvovec), outcomes are substantially better when treatment starts before symptom onset—a rationale that has driven neonatal screening initiatives. Early pathophysiology includes rapid perinatal motor‑neuron loss, reflected by elevated serum neurofilaments and swift declines in compound muscle action potentials (CMAPs). Notably, all three infants with low baseline CMAPs (<1.5 mV) later developed clinically manifest disease, underscoring the prognostic value of early electrophysiologic markers.
Looking ahead, the field aims to define durability and to test sequential/combined strategies. Persistent deficits in some infants with two SMN2 copies raise the question of prenatal intervention. Preclinical data support transplacental or intra‑amniotic approaches, and a single in‑utero risdiplam case (32 weeks’ gestation) remained asymptomatic at 30 months—signals that warrant rigorous clinical evaluation.
For clinicians, these data reinforce two messages: (1) screen early and start therapy promptly, and (2) use objective biomarkers (e.g., neurofilament, CMAP) to anticipate trajectory and tailor follow‑up. Mechanistically, risdiplam exemplifies a gene‑specific, small‑molecule RNA‑processing therapy with potential implications beyond SMA.
.
Editorial note: This content was developed with the support of artificial intelligence technologies to optimize the writing and structuring of the information. All material was carefully reviewed, validated, and supplemented by human experts prior to publication, ensuring scientific accuracy and adherence to good editorial practices.
#SpinalMuscularAtrophy #Risdiplam #NewbornScreening #Neurogenetics #Pediatrics
Sources
- Sumner CJ. Presymptomatic Treatment of a Genetic Disease with a Small Molecule Drug. N Engl J Med. 2025;393(7):715 717. doi:10.1056/NEJMe2507195.
- Finkel RS, Servais L, Vlodavets D, et al. Risdiplam in presymptomatic spinal muscular atrophy. N Engl J Med. 2025;393:671 682




