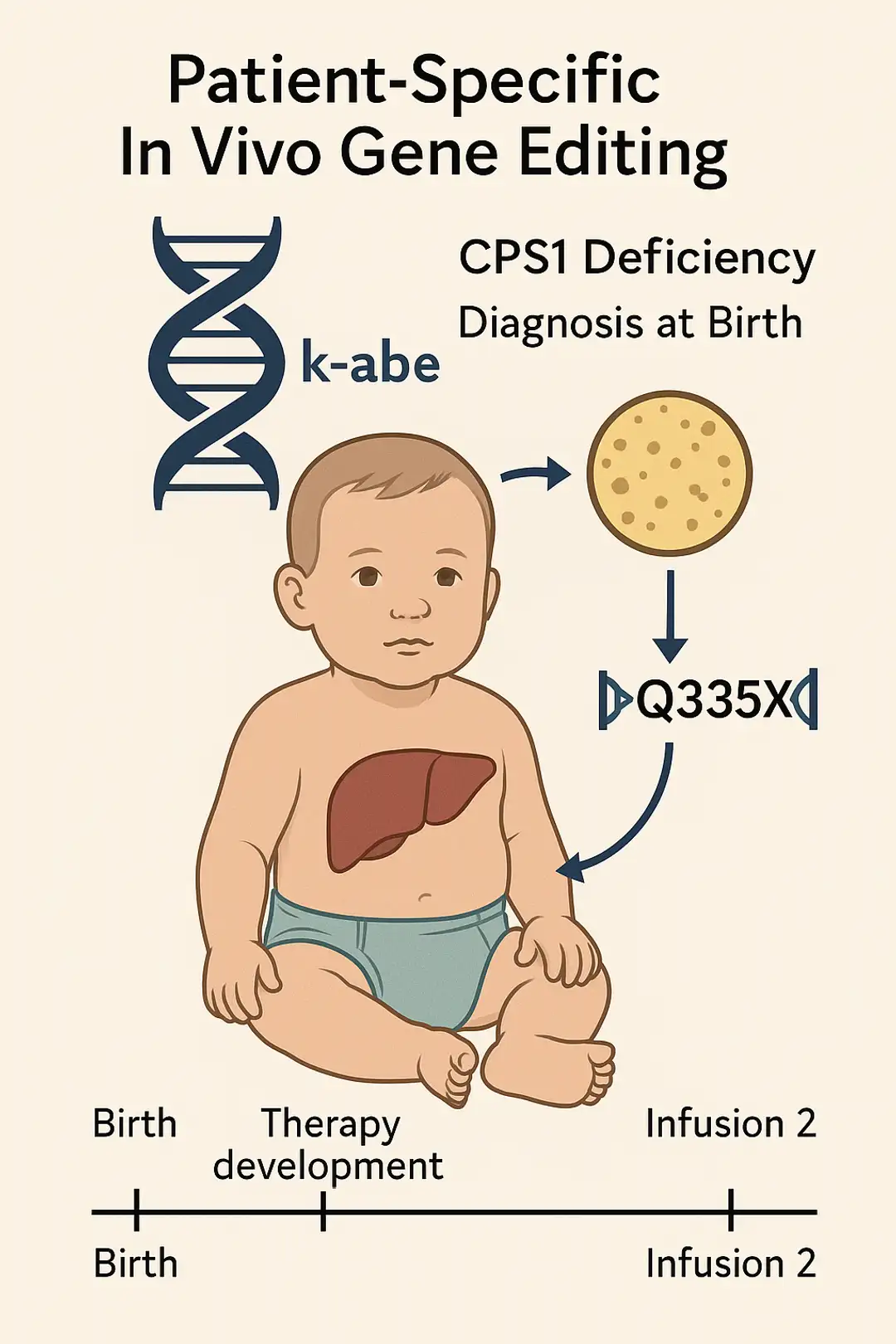
First-in-Human In Vivo Base Editing for CPS1 Deficiency Demonstrates Feasibility of Patient-Customized CRISPR Therapy.
A single-patient expanded-access study showcases the rapid development and safe delivery of lipid nanoparticle–based adenine base editing targeting a rare metabolic disorder.
Carregando conteúdo…
Rare Diseases
Sources
- Musunuru K, Grandinette SA, Wang X, et al. Patient-specific in vivo gene editing to treat a rare genetic disease. N Engl J Med. 2025;390(20):1–9. doi:10.1056/NEJMoa2504747
Highlights
SnackableHealth® |Non-cardiac effects in HELIOS-B trial: gastrointestinal symptoms and quality-of-life signals discussed by Drs. Mike Gibson and Tony Urey
ASCO GU® 2026

Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

SnackableHealth® |Non-cardiac effects in HELIOS-B trial: gastrointestinal symptoms and quality-of-life signals discussed by Drs. Mike Gibson and Tony Urey

ASCO GU® 2026

Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

