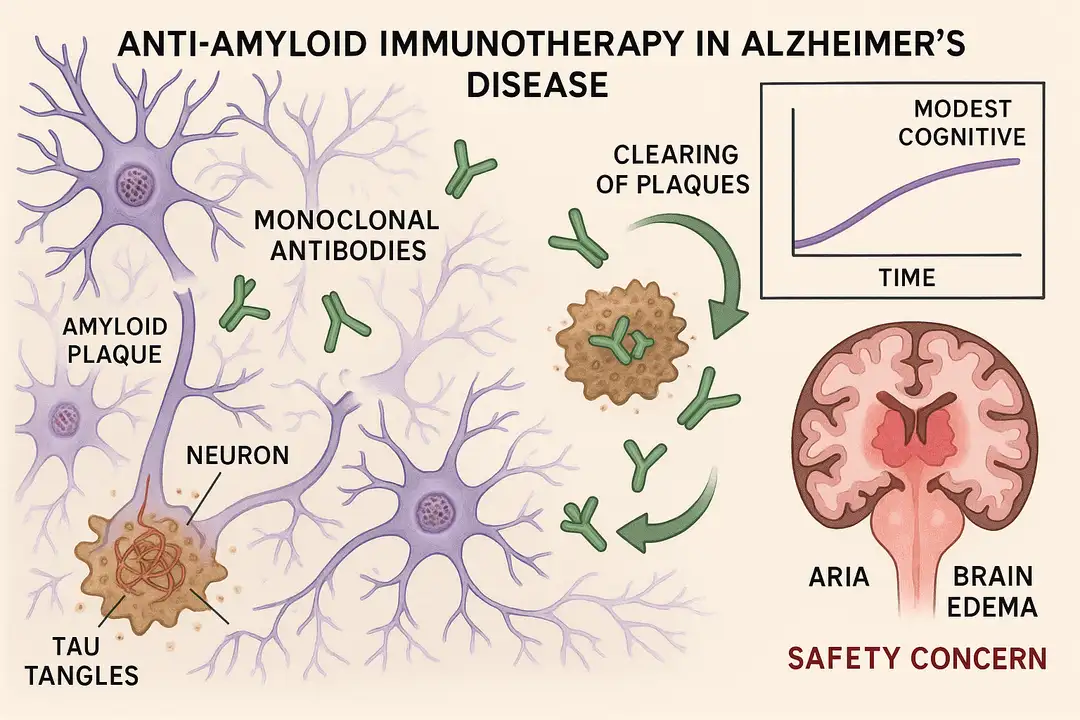
With recent FDA approvals of anti-amyloid beta (anti-Aβ) monoclonal antibodies for Alzheimer's disease, questions remain about their real-world efficacy and safety. This systematic review and meta-analysis examined cognitive outcomes and side effects from phase III trials.
Study Methodology
Researchers analyzed phase III randomized, placebo-controlled trials of anti-Aβ monoclonal antibodies in sporadic Alzheimer's disease. They searched clinicaltrials.gov, PubMed, and Embase through January 2024, focusing on trials with published results in any language.
Primary Outcomes
The analysis examined changes from baseline in two key cognitive measures:
- Clinical Dementia Rating scale-Sum of Boxes (CDR-SB)
- Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog)
Key Findings
Cognitive Benefits:
- Anti-Aβ antibodies showed statistically significant but modest improvements in cognitive decline measures
- Effect sizes were small and of questionable clinical significance
- Benefits were consistent across different antibodies but limited in magnitude
Safety Concerns:
- Increased risk of amyloid-related imaging abnormalities (ARIA)
- Higher rates of brain edema and microhemorrhages
- Some patients required treatment discontinuation due to safety issues
Clinical Significance
While the meta-analysis confirmed statistical benefits of anti-Aβ therapies, the clinical meaningfulness remains debatable. The modest cognitive improvements must be weighed against potential safety risks and high treatment costs.
Study Quality
The analysis included high-quality trials with good Jadad scores. Publication bias assessment using Egger's test and funnel plots showed minimal bias, strengthening the conclusions.
Implications for Practice
The results support FDA approvals but highlight the need for careful patient selection and monitoring. Clinicians should discuss the modest benefits and safety risks with patients and families when considering these therapies.
Future Directions
The findings suggest that anti-amyloid approaches alone may be insufficient for meaningful Alzheimer's treatment. Combination therapies targeting multiple pathways may be necessary for clinically significant improvements.
Patient Selection
The modest benefits emphasize the importance of identifying patients most likely to respond to anti-Aβ therapies, potentially through biomarker-guided treatment approaches.
Editorial note: This content was developed with the support of artificial intelligence technologies to optimize the writing and structuring of the information. All material was carefully reviewed, validated, and supplemented by human experts prior to publication, ensuring scientific accuracy and adherence to good editorial practices.
#antiamyloid #alzheimers #antiabeta #fdaapprovals
Sources
- Tonegawa-Kuji R, Hou Y, Hu B, Lorincz-Comi N, Pieper AA, Tousi B, Leverenz JB, Cheng F. Efficacy and safety of passive immunotherapies targeting amyloid beta in Alzheimer's disease: A systematic review and meta-analysis. PLoS Med. 2025 Mar 31;22(3):e1004568. doi: 10.1371/journal.pmed.1004568. PMID: 40163534; PMCID: PMC12002640.
Highlights
Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care
Medical Affairs
META-AF: Metformin as an Adjunctive Therapy to Catheter Ablation of Atrial Fibrillation

Medical Affairs
Trastuzumab deruxtecan (T-DXd) Provides Significant Clinical Benefit Over Trastuzumab emtansine (T-DM1), Marking a Potential Shift in the Therapeutic Standard for HER2+ Breast Cancer

TribeMD
SnackableHealth™ | From Intervention to Prevention: How Secondary Prevention Clinics Are Redefining Post-ACS Care

Medical Affairs
META-AF: Metformin as an Adjunctive Therapy to Catheter Ablation of Atrial Fibrillation


